PUBLICATIONS and PATENTS
70.
Austin M. Stanley, Christopher Tremblay, Nathan D. Schley, Heather L. Buckley, David C. Leitch,* and Erin T. Chernick.
J. Org. Chem. 2026, in revision.
ChemRxiv 2025, DOI: 10.26434/chemrxiv-2025-zbwq6.
69.
Kushal D. Dhake and David C. Leitch.*
Introducing the heterocyclic[3.1.1]propellanes
Nature Chemistry 2026, DOI: 10.1038/s41557-026-02074-0.
News & Views.
68.
Paul Clark, Thomas Keenan, Shiqi Liu, Jingjun Huang, Yao-Rui Ma, Avantika Hasija, Pei-Qiang Huang, Alexandre Jean, David C. Leitch,* and Stellios Arseniyadis.*
Chem. Commun. 2025, 61, 19064-19067.
67.
Ian C. Chaguna, Antonia Kropp, David C. Leitch,* and J. Scott McIndoe.*
Organometallics 2025, 44, 628-636.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-nx480.
66.
Gregory Gaube, Douglas L. Miller, Rowan A. McCallum, Nahiane Pipaon Fernandez, and David C. Leitch.*
Tetrahedron 2025, 182, 134682.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-5mlml.
65.
Kushal Dhake, Kyla J. Woelk, Liam D. N. Krueckl, Faith Alberts, James Mutter, Matthew O. Pohl, Gilian T. Thomas, Muskan Sharma, Jaelyn Bjornerud-Brown, Nahiane Pipaon Fernandez, Nathan D. Schley, and David C. Leitch.*
Chem. Commun. 2024, 60, 13008-13011.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-59vs5.
64.
Gilian T. Thomas, Odhran D. Cruise, Daelin Peel-Smith, Nahiane Pipaon Fernandez, Charles Killeen, and David C. Leitch.*
Easily Accessible and Solution-Stable Ni(0) Precatalysts for High-Throughput Experimentation
Chem. Eur. J. 2025, 31, e202403960.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-qpdc6-v2.
63.
Jingru Lu, Holly Celuszak, Irina Paci,* and David C. Leitch.*
Chem. Eur. J. 2024, 30, e202402283.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-zfjp6.
62.
Jingru Lu, Holly Celuszak, Irina Paci,* and David C. Leitch.*
Chem. Eur. J. 2024, 30, e202402282.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-v3q40.
61.
Jingru Lu, Nathan D. Schley, Irina Paci,* and David C. Leitch.*
Organometallics 2024, 43, 3192-3203.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-lrdz4-v2.
60.
Gilian T. Thomas, Jared Z. Litman, Binh Dang Ho, Jingjun Huang, Kalina Blonska, Nathan D. Schley, and David C. Leitch*
Organometallics 2024, 43, 2413-2426.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-g4vxk.
59.
Jingjun Huang, Binh Dang Ho, Gregory Gaube, Holly Celuszak, Joseph Becica, Nathan D. Schley, and David C. Leitch*
Organometallics 2024, 43, 2403-2412.
ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-ml728.
58.
David C. Leitch* and Gilian T. Thomas
eEROS 2024, DOI: 10.1002/047084289X.rn02576.
57.
Nahiane Pipaon Fernandez, Odhran Cruise, Sarah E. F. Easton, Justin M. Kaplan, John L. Woodard, Damian P. Hruszkewycz,* and David C. Leitch*
J. Org. Chem. 2024, 89, 16145-16160.
ChemRxiv 2023, DOI: 10.26434/chemrxiv-2023-2ffdr.
56.
Julia Levy, Ian C. Chagunda, Violeta Iosub, David C. Leitch, and J. Scott McIndoe*
MoleculAR: An Augmented Reality Application for Understanding 3D Geometry
J. Chem. Ed. 2024, 101, 2533-2539.
55.
Gregory Gaube, James Mutter, and David C. Leitch*
A “neat” synthesis of substituted 2-hydroxy-pyrido[1,2-a]pyrimidin-4-ones
Can. J. Chem. 2024, 102, 206-213.
ChemRxiv 2023, DOI: 10.26434/chemrxiv-2023-09l28.
54.
Sydney Reiber, Bennett Templeman-Vivian, Michelle B. Mills, Christopher Tremblay, David C. Leitch,* and Erin T. Chernick
Multiple verdazyl radicals appended to a triarylamine scaffold
Can. J. Chem. 2024, 102, 99-107.
ChemRxiv 2023, DOI: 10.26434/chemrxiv-2023-7dc4s.
53.
Kyla J. Woelk, Kushal Dhake, Nathan D. Schley, and David C. Leitch*
Enolate addition to bicyclobutanes enables expedient access to 2-oxo-bicyclohexane scaffolds
Chem. Commun. 2023, 59, 13847-13850.
ChemRxiv 2023, DOI: 10.26434/chemrxiv-2023-vt063-v2.
52.
Jingru Lu and David C. Leitch*
ACS Catal. 2023, 13, 15691-15707.
ChemRxiv 2023, DOI: 10.26434/chemrxiv-2023-2078b-v2.
51.
Jingjun Huang, Thomas Keenan, Francois Richard, Jingru Lu, Sarah E. Jenny, Alexandre Jean, Stellios Arseniyadis,* and David C. Leitch*
Nature Commun. 2023, 14, 8058.
ChemRxiv 2022, DOI: 10.26434/chemrxiv-2023-0xbh5.
50.
David Leitch, Jingjun Huang, and Stellios Arseniyadis
World Patent Application WO2023156972A1, Published 2023.
49.
David C. Leitch*
The Power of High Throughput Experimentation: General Topics and Enabling Technologies for Synthesis and Catalysis 2022, 1, 35-57. ACS Symposium Series.
48.
Jingru Lu, Irina Paci,* and David C. Leitch*
Chem. Sci. 2022, 13, 12681-12695.
ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-71bzc.
47.
François Richard, Sidonie Aubert, Tania Katsina, Luuk Reinalda, David Palomas, Rachel Crespo-Otero, Jingjun Huang, David C. Leitch, Carlos Mateos, and Stellios Arseniyadis*
Nature Synth. 2022, 1, 641-648.
46.
Erin. G. Moloney, Deepak. T. Gangadharan, Vishal Yeddu, Dongyang Zhang, Shahram Moradi, Abdelrahman M. Askar, Michael. M. Adachi, David C. Leitch, and Makhsud I. Saidaminov.*
Chem. Mater. 2022, 34, 4394-4402.
45.
David Leitch, Jingjun Huang, Matthew Isaac, Ryan Watt
Palladium complex and catalyst embodiments and methods of making and using the same
World Patent Application WO2022153180A1, Published 2022.
44.
David C. Leitch* and Joseph Becica
High-Throughput Experimentation in Organometallic Chemistry and Catalysis
Comprehensive Organometallic Chemistry IV 2022, 13, 502-555.
43.
Nahiane Pipaon Fernandez, Gregory Gaube, Kyla J. Woelk, Mathias Burns, Damian P. Hruszkewycz, and David C. Leitch*
ACS Catal. 2022, 12, 6997-7003.
ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-s62r6.
42.
Kushal Dhake, Kyla J. Woelk, Joseph Becica, Andy Un, Sarah E. Jenny, and David C. Leitch*
Angew. Chem. Int. Ed. 2022, 61, e202204719.
ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-p0fzf.
41.
Jingru Lu, Sofia Donnecke, Irina Paci,* and David C. Leitch*
Chem. Sci. 2022, 13, 3477-3488.
ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-qc8vv-v2.
40.
Gregory Gaube, Nahiane Pipaon Fernandez, and David C. Leitch*
An evaluation of palladium-based catalysts for the base-free borylation of alkenyl carboxylates.
New J. Chem. 2021, 45, 20095-20098.
ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-x0h4m-v2.
39.
Joseph Becica, Owen D. Glaze, Damian P. Hruszkewycz,* Graham E. Dobereiner,* and David C. Leitch*
React. Chem. Eng. 2021, 6, 1212-1219.
38.
Emma Dennis, Victor Burlakov, Jingjun Huang, Soumya Kundu, Deepak Thrithamarassery Gangadharan, Devon Richtsmeier, Magdalena Bazalova-Carter, David C. Leitch, and Makhsud I. Saidaminov*
CrystEngComm 2021, 23, 2215-2221.
37.
Jingjun Huang, Matthew Isaac, Ryan Watt, Joseph Becica, Emma Dennis, Makhsud I. Saidaminov, William A. Sabbers, and David C. Leitch*
ACS Catalysis 2021, 11, 5636–5646.
ChemRxiv 2021, DOI: 10.26434/chemrxiv.13604777.v1.
36.
Joseph Becica and David C. Leitch*
C-O Bond Activation as a Strategy in Palladium-Catalyzed Cross-Coupling.
Synlett 2021, 32, 641-646.
Invited Synpact account.
35.
Joseph Becica, Oliver R. J. Heath, Cameron H. M. Zheng, and David C. Leitch*
Palladium-Catalyzed Cross-Coupling of Alkenyl Carboxylates.
Angew. Chem. Int. Ed. 2020, 59, 17277-17281.
34.
Joseph Becica, Gregory Gaube, William A. Sabbers, and David C. Leitch*
Dalton Trans. 2020, 49, 16067-16071.
Part of the special issue New Talent: Americas
D. C. Leitch GSK Research:
33.
John Jin Lim,* Frank Dixon Jr., David C. Leitch, John Kowalski, Mark Nilson, Charles Goss, Roy Flanagan, Sean Hayes, and Michael J. Murphy
Playing with Fire? A Safe and Effective Deactivation of Raney Cobalt using Aqueous Sodium Nitrate.
Org. Process Res. Dev. 2020, 24, 1180-1184.
32.
John Jin Lim,* Kenneth Arrington,* Anna L. Dunn, David C. Leitch, Ian Andrews, Neil R Curtis, Mark J. Hughes, Daniel Tray, Charles E. Wade, Matthew P. Whiting, Charles Goss, Yangmu Chloe Liu, and Brian Roesch
Org. Process Res. Dev. 2020, 24, 1927-1937.
31.
Joseph Becica, Damian P. Hruszkewycz, Janelle E. Steves, Jennifer M. Elward, David C. Leitch,* and Graham E. Dobereiner*
Org. Lett. 2019, 21, 8981-8986.
ChemRxiv, DOI: 10.26434/chemrxiv.9108461
30.
Jonathan D. Egbert, Edwin C. Thomsen, Stacy A. O'Neill-Slawecki, Douglas M. Mans, David C. Leitch, Lee J. Edwards, Charles E. Wade, and Robert S. Weber*
Org. Process Res. Dev. 2019, 23, 1803-1812.
Gregg A. Barcan, Jose J Conde, Mohamed K. Mokhallalati, Mark G. Nilson, Shiping Xie,* C. Liana Allen, Yemane W. Andemichael, Nicholas A. Calandra, David C. Leitch, Ling Li, and Michael J. Morris
Org. Process Res. Dev. 2019, 23, 1396-1406.
Steven M. Mennen,* Carolina Alhambra, C. Liana Allen, Mario Barberis, Simon Berritt, Thomas A. Brandt, Andrew D. Campbell, Jesús Castañón, Alan H. Cherney, Melodie Christensen, David B. Damon, J. Eugenio de Diego, Susana García-Cerrada, Pablo García-Losada, Rubén Haro, Jacob Janey, David C. Leitch, Ling Li, Fangfang Liu, Paul C. Lobben, David W. C. MacMillan, Javier Magano, Emma McInturff, Sebastien Monfette, Ronald J. Post, Danielle Schultz, Barbara J. Sitter, Jason M. Stevens, Iulia I. Strambeanu, Jack Twilton, Ke Wang, Matthew A. Zajac
Org. Process Res. Dev. 2019, 23, 1213-1242.
Selected as an ACS Editors' Choice Paper (open access).
Kenneth Arrington, Gregg A. Barcan, Nicholas A. Calandra, Greg A. Erickson, Ling Li, Li Liu, Mark G. Nilson, Iulia I. Strambeanu, Kelsey F. VanGelder, John L. Woodard, Shiping Xie, C. Liana Allen, John A. Kowalski,* and David C. Leitch*
Convergent Synthesis of the NS5B Inhibitor GSK8175 Enabled by Transition Metal Catalysis.
J. Org. Chem. 2019, 84, 4680-4694.
Part of the special issue Excellence in Industrial Organic Synthesis 2019.
C. Liana Allen, David C. Leitch, Michael S. Anson, and Matthew A. Zajac*
The power and accessibility of high-throughput methods for catalysis research.
Nature Catalysis 2019, 2, 2-4.
Anna L. Dunn,* David C. Leitch,* Michel Journet, Michael Martin, Elie A. Tabet, Neil R. Curtis, Glynn Williams, Charles Goss, Tony Shaw, Bernie O’Hare, Charles Wade, Matthew A. Toczko, and Peng Liu
Organometallics 2019, 38, 129-137.
Part of the special issue The Roles of Organometallic Chemistry in Pharmaceutical Research and Development.
Justin M. Kaplan, Damian P. Hruszkewycz, Iulia I. Strambeanu, Christopher J. Nunn, Kelsey F. VanGelder, Anna L. Dunn, Derek I. Wozniak, Graham E. Dobereiner, and David C. Leitch*
Organometallics 2019, 38, 85-96.
Part of the special issue The Roles of Organometallic Chemistry in Pharmaceutical Research and Development.
Julien C. Vantourout, Ling Li, Enrique Bendito-Moll, Bela E. Bode, Mark G. Nilson, John L. Woodard, Albert Isidro-Llobet, Katherine M. P. Wheelhouse, David C. Leitch,* and Allan J. B. Watson*
ACS Catalysis 2018, 8, 9560-9566.
John Jin Lim,* and David C. Leitch
Org. Process Res. Dev. 2018, 22, 641-649.
David C. Leitch, Jay A. Labinger, John E. Bercaw, and Yan C. Lam
Method for the Conversion of Hydrocarbons via Tandem Transfer Hydrogenation and Oligomerization.
US Patent 9,676,680. Granted June 2017.
David C. Leitch,* Matthew P. John, Paul A. Slavin, and Andrew D. Searle
Org. Process Res. Dev. 2017, 21, 1815-1821.
David C. Leitch,* Thomas F. Greene, Roisin O’Keeffe, Thomas C. Lovelace, Jeremiah D. Powers, and Andrew D. Searle
Org. Process Res. Dev. 2017, 21, 1806-1814.
D. C. Leitch Supervised Research:
Huseyin Erguven, David C. Leitch, Evan N. Keyzer, and Bruce A. Arndtsen*
Development and Cycloaddition Reactivity of a New Class of Pyridine-Based Mesoionic 1,3-Dipole.
Angew. Chem. Int. Ed. 2017, 56, 6078-6082.
Part of the special issue 100th Anniversary of the Canadian Society for Chemistry.
David C. Leitch, Laure Kayser, Zhi-Yong Han, Ali R. Siamaki, Evan N. Keyzer, Ashley Gefen, and Bruce A. Arndtsen*
Nature Commun. 2015, 6, 7411.
Patricia Horrillo-Martinez, David C. Leitch, Scott A. Ryken, Robert K. Thomson, J. David Beard, Brian O. Patrick, and Laurel L. Schafer*
Can. J. Chem. 2015, 93, 775-783.
Jay A. Labinger,* David C. Leitch, John E. Bercaw, Mark A. Deimund, and Mark E. Davis
Upgrading Light Hydrocarbons: A Tandem Catalytic System for Alkane/Alkene Coupling.
Top. Catal. 2015, 58, 494-501.
David C. Leitch, Jay A. Labinger,* and John E. Bercaw*
Organometallics 2014, 33, 3353-3365.
Jacky C.-H. Yim, Jason A. Bexrud, Rashidat O. Ayinla, David C. Leitch, and Laurel L. Schafer*
J. Org. Chem. 2014, 79, 2015-2028.
Renato Skerlj* et al.
J. Med. Chem. 2013, 56, 8049-8065.
David C. Leitch, Yan C. Lam, Jay A. Labinger,* and John E. Bercaw*
J. Am. Chem. Soc. 2013, 135, 10302-10305.
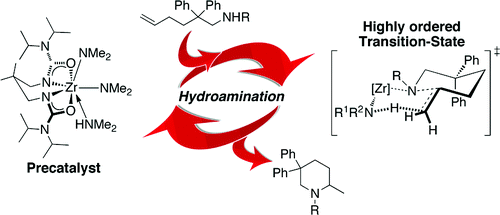
David C. Leitch, Rachel H. Platel, and Laurel L. Schafer* Mechanistic Elucidation of Intramolecular Aminoalkene Hydroamination Catalyzed by a Tethered Bis(ureate) Complex: Evidence for Proton-Assisted C-N Bond Formation at Zirconium.
J. Am. Chem. Soc. 2011, 133, 15453-15463.
Philippa R. Payne, Jason A. Bexrud, David C. Leitch, and Laurel L. Schafer*
Can. J. Chem. 2011, 89, 1222-1229.
David C. Leitch, Courtney S. Turner, and Laurel L. Schafer*
Angew. Chem. Int. Ed. 2010, 49, 6382-6386.
David C. Leitch and Laurel L. Schafer*
Organometallics 2010, 29, 5162-5172.
Part of the Dietmar Seyferth Festschrift.
David C. Leitch, Philippa R. Payne, Christine R. Dunbar, and Laurel L. Schafer*
Broadening the Scope of Group 4 Hydroamination Catalysis using a Tethered Ureate Ligand.
J. Am. Chem. Soc. 2009, 131, 18246-18247.
Jason A. Bexrud, Patrick Eisenberger, David C. Leitch, Philippa R. Payne, and Laurel L. Schafer*
J. Am. Chem. Soc. 2009, 131, 2116-2118.
David C. Leitch, J. David Beard, Robert K. Thomson, Vincent A. Wright, Brian O. Patrick, and Laurel L. Schafer*
Eur. J. Inorg. Chem. 2009, 2691-2701.
Mark C. Wood, David C. Leitch, Charles S. Yeung, Jennifer A. Kozak, and Laurel L. Schafer*
Chiral Neutral Zirconium Amidate Complexes for the Asymmetric Hydroamination of Alkenes.
Angew. Chem. Int. Ed. 2007, 46, 354-358.
Zhe Zhang, David C. Leitch, Man Lu, Brian O. Patrick, and Laurel L. Schafer*
An Easy-To-Use, Regioselective, and Robust Bis(Amidate) Titanium Hydroamination Precatalyst: Mechanistic and Synthetic Investigations Toward the Preparation of Tetrahydro-isoquinolines and Benzoquinolizine Alkaloids.
Chem. Eur. J. 2007, 13, 2012-2022.
Jason A. Bexrud, J. David Beard, David C. Leitch, and Laurel L. Schafer*
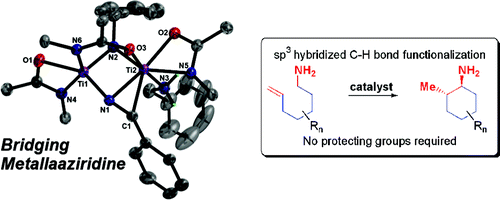
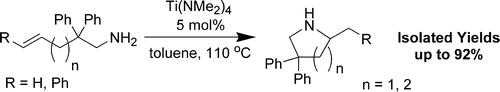
Intramolecular Hydroamination of Unactivated Olefins with Ti(NMe2)4 as a Precatalyst.
Org. Lett. 2005, 7, 1959-1962.